Review Modifications of Human Serum Albumin and Their Binding Effect
Serum Albumin
Structure
Serum albumin is the nigh arable protein in the blood plasma of all vertebrates with the concentration in human serum beingness 35–fifty mg/mL (Peters, 1996). Human being serum albumin (HSA) has a molecular mass of 66,348 Da and is equanimous of three homologous domains, numbered I, II, and III (Effigy one) (He and Carter, 1992; Peters, 1996; Sugio et al., 1999). Each domain is grouped into subdomains A and B that possess mutual structural motifs. The two principal regions responsible for ligand-binding to HSA are known as Sudlow'southward Site I and II, located in subdomain IIA and IIIA (Figure i), respectively (Sudlow et al., 1976; Peters, 1996). Albumin is coded by a single factor, which is expressed in a co-dominant mode with both alleles existence transcribed and translated (Hawkins and Dugaiczyk, 1982; Peters, 1996). The homo albumin factor is located on the long arm of chromosome four at position q13.3.
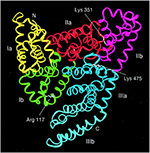
Figure ane. Structure of man serum albumin consisting of three domains, each grouped into subdomains A and B (Subdomain Ia, yellowish; Ib, green; IIa, red; IIb, magenta; IIIa, blue; and IIIb, cyan). Sugio et al. (1999) past permission of Oxford University Press.
Part
Albumin is primarily synthesized by the liver with the human liver producing ~13.ix g of HSA per day (Peters, 1996). HSA has an approximate half-life of 19 days and is degraded more finer if it is denatured or structurally contradistinct (Peters, 1996). Albumin has a multifariousness of important functions and is responsible for fourscore% of the colloidal osmotic pressure of blood (Peters, 1996). Significantly, albumin is able to bind various endogenous molecules, including long-chain fatty acids, steroids, L-tryptophan, etc. (Kragh-Hansen, 1981; Peters, 1996; Evans, 2002). Moreover, albumin is also involved in transporting ions in the circulation, including copper, zinc, calcium, etc. (Peters, 1996).
Additionally, this vital protein is able to bind exogenous compounds and drugs, such as warfarin, ibuprofen, chlorpromazine and naproxen, with the analogousness of their bounden significantly affecting their activity and half-life (Kragh-Hansen, 1981; Peters, 1996; Evans, 2002). Furthermore, albumin also acts equally a toxic waste carrier, binding bilirubin, the product of heme breakdown, to deliver information technology to the liver for hepatic excretion (Peters, 1996). Interestingly, albumin is also believed to act as an anti-oxidant on business relationship of its ability to: (1) protect bound substances from peroxidative harm (e.g., fat acids and lipoproteins); and (ii) bind gratis copper, limiting its redox action and the product of free radicals (Peters, 1996; Evans, 2002). Lastly, albumin is a source of thiols that are avid reactive oxygen and nitrogen species scavengers (Peters, 1996; Evans, 2002).
Distribution
Interestingly, albumin is predominately present in the extravascular space (~242 chiliad) rather than the intravascular infinite (~118 chiliad) (Peters, 1996; Evans, 2002). In fact, the poly peptide is prevalent in extracellular locations such as peel, gut, muscle, other fluids (i.e., cerebrospinal, pleural, etc.) and secretions (e.g., sweat, tears and milk) (Peters, 1996). Still, very low concentrations of albumin are present intracellularly (Peters, 1996). Albumin returns from the extravascular space to the circulation via the lymphatic system, making ~28 "trips" in and out of the lymphatic system during its lifetime (Peters, 1996; Evans, 2002).
Upon secretion from hepatocytes, albumin enters the circulation and translocates to the extracellular space through the pores of sinusoidal or fenestrated endothelium in sure organs, such every bit the liver, pancreas, pocket-size intestine and bone marrow (Peters, 1996). Nevertheless, in organs where a continuous endothelium predominates, it is now believed that albumin tin can traverse the endothelium via active transcytotic mechanisms, including receptor-mediated mechanisms (eastward.grand., albondin; meet Section entitled "Cellular Albumin-Binding Proteins").
Accumulation of Albumin in the Tumor Interstitium
Solid tumors commonly possess an immature, highly permeable vasculature that is acted upon by vascular permeability-enhancing factors (e.g., nitric oxide) (Carmeliet and Jain, 2000; Maeda et al., 2000; Greish, 2007; van der Veldt et al., 2008). Notwithstanding, despite this in that location is generally insufficient lymphatic drainage (Carmeliet and Jain, 2000; Maeda et al., 2000; Greish, 2007). This later results in an accumulation of macromolecules (>40 kDa) within the tumor interstitium, and this is known equally the enhanced permeation and memory effect (Figure 2) (Maeda et al., 2000; Greish, 2007). Of involvement, Matsumura and Maeda (1986) demonstrated that an intravenously injected Evans blue-albumin complex accumulated in sarcoma 180 tumors of ddY mice. The memory of albumin in tumors has since been observed in diverse experimental solid tumors (e.g., sarcoma, ovarian carcinoma, Novikof hepatoma, etc.) using radiolabeled- or dye-complexed serum albumin (Peterson and Appelgren, 1973; Sinn et al., 1990; Andersson et al., 1991; Schilling et al., 1992; Stehle et al., 1997; Wunder et al., 1997).
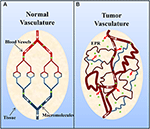
Effigy two. Schematic representation of (A) normal and (B) tumor vasculature. Normal tissue is composed of mature, organized blood vessels, while tumor tissue consists of young, leaky and tortuous vessels. The contradistinct organization of tumor vasculature and disorganized lymphatic network results in vascular leakage and the accumulation of macromolecules (>40 kDa) within the interstitium and is known as the enhanced permeation and retention (EPR) outcome. Adapted by permission from Macmillan Publishers Ltd: Nature Medicine (Jain, 2001), copyright (2001).
Additionally, a number of studies have proposed that tumors are a site of albumin catabolism (Hradec, 1958; Andersson et al., 1991; Schilling et al., 1992; Stehle et al., 1997). For instance, in a mouse sarcoma model (C57/RL6J) injected with 3H-raffinose-labeled albumin, at least 2–3-fold greater levels of 3H were observed in the lysosomes of tumors when compared with lysosomes of normal tissue (Andersson et al., 1991). Furthermore studies have demonstrated that albumin has a shorter half-life and a higher turnover in tumor-bearing mice, despite a compensatory increase in hepatic albumin synthesis, compared to non-tumor-bearing mice (Hradec, 1958). Hence, it has been suggested that tumors utilize albumin as a source of free energy, by breaking down albumin into its component amino acids in lysosomes that are subsequently used by cancer cells for their accelerated growth (Stehle et al., 1997). Moreover, studies have suggested that the hypoalbuminiemia evident in cancer patients is a issue of albumin catabolism by the tumor (Stehle et al., 1997).
Nevertheless, some of these before studies suffer from several experimental limitations. For example, it is difficult to obtain pure lysosomal fractions and, thus, it is necessary to reproduce these studies and exam the purity of fractions using well established membrane and organelle markers (Graham, 2002; Yamagishi et al., 2013). Additionally, several other in vivo factors may affect albumin degradation and catabolism (e.g., levels of corticosteroids) (Peters, 1996). Therefore, a articulate-cut relationship has not been established and additional in vivo studies are necessary to support the intracellular distribution and catabolism of albumin by tumors.
More recently, Commisso et al. observed that cancer cells harboring endogenous oncogenic Ras mutations accept increased levels of macropinocytosis in vitro and in vivo (Commisso et al., 2013). Moreover, it was demonstrated that FITC-labeled albumin was internalized through macropinocytosis and subsequently resulted in increased levels of glutamate and α-ketoglutarate in oncogenic Ras-transformed cells (Commisso et al., 2013). Interestingly, the decrease in proliferation of oncogenic Ras-expressing cells after glutamine impecuniousness was shown to exist rescued by extracellular albumin supplementation (Commisso et al., 2013). These findings suggest that macropinocytosis of albumin provides nutrients to sustain cancer cell proliferation (Commisso et al., 2013).
Cellular Albumin-Binding Proteins
Considering the importance of albumin, a number of putative albumin-binding proteins and receptors have been identified in various tissues and cell lines (Table one), including kidney (Zhai et al., 2000; Amsellem et al., 2010), endothelium (Schnitzer and Bravo, 1993), fibroblasts (Porter et al., 1995), and tumor-cell surfaces (Fritzsche et al., 2004). Specifically, seven membrane-associated albumin-binding proteins accept been discovered, namely: albondin/glycoprotein lx (gp60) (Schnitzer et al., 1988), glycoprotein 18 (gp18) (Ghinea et al., 1988), glycoprotein 30 (gp30) (Ghinea et al., 1988), the neonatal Fc receptor (FcRn) (Roopenian and Akilesh, 2007), heterogeneous nuclear ribonucleoproteins (hnRNPs) (Fritzsche et al., 2004), calreticulin (Fritzsche et al., 2004), cubilin (Zhai et al., 2000; Amsellem et al., 2010), and megalin (Zhai et al., 2000; Amsellem et al., 2010). Moreover, a secreted albumin-binding protein known as secreted poly peptide, acidic and rich in cysteine (SPARC) has been identified (Schnitzer and Oh, 1992). Because their importance in albumin uptake past cells, each of these proteins are described in detail beneath.

Tabular array 1. Localization of albumin-binding proteins and receptors.
Albondin/gp60
Albondin (gp60) is a sixty kDa glycoprotein that acts equally an albumin receptor that is widely distributed, just is selectively expressed on the plasma membrane of continuous endothelium (except for the encephalon), where information technology operates to increase capillary permeability (Ghinea et al., 1988, 1989; Schnitzer et al., 1988; Schnitzer, 1992; Schnitzer and Oh, 1994; Tiruppathi et al., 1996). Albondin non but specifically binds native albumin, merely also facilitates its internalization and subsequent transcytosis (Milici et al., 1987; Schnitzer, 1992; Schnitzer and Oh, 1994; Tiruppathi et al., 1996).
It has been proposed that ~l% of albumin leaves the capillary lumen via albondin, with the remainder traversing this bulwark through intercellular junctions and/or fluid-phase mechanisms (Schnitzer, 1993; Schnitzer and Oh, 1994). Moreover, it has been demonstrated that the internalization of albondin occurs through a caveolin-dependent endocytotic process that results in transcytosis and does not appear to enter the degradative endosome-lysosome system (Schnitzer, 1993; Schnitzer and Bravo, 1993; Schnitzer et al., 1995; Tiruppathi et al., 1997; Iancu et al., 2011).
gp18 and gp30
Both gp18 and gp30 avidly demark conformationally-modified albumin (i.e., gilt-labeled albumin, formaldehyde- or maleic-anhydride-treated albumin) and practise not preferentially interact with native albumin, similarly to other known scavenger receptors (Ghinea et al., 1989; Schnitzer and Oh, 1992, 1994; Schnitzer et al., 1992; Schnitzer and Bravo, 1993). Different albondin, gp18 and gp30 are found on a diverseness of cells, such equally macrophages and fibroblasts, and a range of endothelia (Schnitzer et al., 1992). Moreover, gp18 has been observed to exist expressed in homo MDA-MB-453 chest cancer cells (Wang et al., 1994). These scavenger receptors bind and straight modified albumins for degradation, perhaps as part of protein catabolism or as a protective pathway to remove altered, old, damaged or potentially deleterious albumins (Schnitzer, 1993; Schnitzer and Bravo, 1993). Albumin may exist modified through oxidation, not-enzymatic glycation, maleylation, etc. as a result of normal aging or as a protective or pathological response (Schnitzer, 1993; Peters, 1996). Denatured or modified albumin is degraded faster and more efficiently than native albumin, suggesting that these alterations select albumin molecules for degradation (Peters, 1996).
SPARC
SPARC is also known every bit osteonectin and BM-40 and is secreted by several cell types (Brekken and Sage, 2001). Interestingly, SPARC has been found to be highly expressed in malignant cells and stromal cells associated with neoplasia (Porter et al., 1995; Podhajcer et al., 2008). SPARC possesses albumin-binding properties and specifically interacts with native albumin in a like way to albondin, merely differing from eighteen to gp30 that demark conformationally altered albumin (Schnitzer and Oh, 1992). Specifically, anti-SPARC antibodies also recognize albondin, but not xviii or gp30, suggesting that SPARC and albondin share a native albumin-binding domain (Schnitzer and Oh, 1992). However, at that place is no show that SPARC mediates albumin uptake into tumors. Information technology has been postulated that the ability of SPARC to bind albumin in the tumor interstitium enhances the accumulation of albumin-jump drugs within the tumor space (Desai et al., 2008, 2009). Moreover, a preliminary clinical trial demonstrated that SPARC expression correlated with the response to paclitaxel-loaded albumin nanoparticle (nab-paclitaxel or Abraxane®) treatment, with SPARC-positive patients having a better clinical outcome (Desai et al., 2009). However, conflicting data in a KPfC mouse model has challenged this hypothesis, equally SPARC deficiency did non change the intra-tumoral concentrations of Abraxane® (Neesse et al., 2014). Consequently, farther studies are necessary to validate this hypothesis, including larger clinical trials involving a greater number of patients. Currently, a phase Iii study (NCT00785291), past the National Cancer Found, is evaluating whether SPARC expression in serum predicts patient response to Abraxane®, and this may further our understanding of the role of SPARC in albumin accumulation past tumors.
hnRNPs and Calreticulin
V different albumin-binding proteins have been identified from plasma membranes of human cancer cells lines (i.e., CCRF-CEM T-cell leukemia, MV3 melanoma and MCF7 chest carcinoma) (Fritzsche et al., 2004). Iv of these proteins were identified every bit members of the hnRNP family, including hnRNP A2/B1, hnRNP C1, hnRNP A1 and hnRNP A3, and the fifth poly peptide was plant to be calreticulin (Fritzsche et al., 2004). Calreticulin was outset described every bit an endoplasmic reticulum chaperone and calcium signaling protein, only has since been shown to be involved in several cellular functions, including cell adhesion, modulation of platelet-collagen interactions (wound healing) and apoptosis (Mendlovic and Conconi, 2010). The functions of the hnRNP family are not well characterized. However, most members of the hnRNP family unit have been described equally nuclear RNA-binding proteins involved in pre-mRNA processing, such as RNA splicing, export and stability (Chaudhury et al., 2010). Interestingly, hnRNPs have been proposed to play a office in carcinogenesis where their over-expression acts as biomarkers for the early detection of tumors (Han et al., 2013). The significance of these findings is currently unclear and it remains to exist determined whether these proteins are involved in albumin-mediated uptake.
FcRn
FcRn is expressed in multiple cell-types and tissues, including antigen-presenting cells, vascular endothelium, gut, lungs, kidneys and the claret-brain barrier (BBB) (i.e., endothelium and choroid plexus) (Roopenian and Akilesh, 2007). This receptor protects albumin and IgG, from degradation by binding both proteins with high affinity only at a depression pH (pH < 6.v) in acidic endosomes, preventing their degradation via the lysosomal pathway and returning them to the extracellular space (pH 7.iv) (Chaudhury et al., 2003; Ober et al., 2004; Anderson et al., 2006; Andersen et al., 2012). This consequently extends the half-life of serum albumin (Chaudhury et al., 2003; Anderson et al., 2006; Sarav et al., 2009). The office of this receptor in albumin uptake by tumors remains unclear.
Cubilin and Megalin
Cubilin is a multi-ligand receptor that is most recognized for its involvement in the intestinal uptake of the intrinsic cistron vitamin B12 complex (Seetharam et al., 1997). Moreover, cubilin has been shown to be involved in the endocytosis and transcellular ship of numerous ligands, including albumin (Birn et al., 2000). Cubilin is localized to absorptive intestinal cells, placenta, visceral yolk-sac cells and proximal tubules of kidneys (Christensen and Birn, 2002). Megalin is a big trans-membrane protein that has also been shown to bind albumin (Cui et al., 1996). This protein is more widely expressed than cubilin, being present in the choroid plexus, kidney proximal tubule cells, thyrocytes, etc. (Tabular array 1) (Christensen and Birn, 2002).
Interestingly, megalin binds to cubilin with high analogousness and it has been suggested that megalin contributes to the internalization of cubilin-ligand complexes every bit a co-receptor (Moestrup et al., 1998; Christensen and Birn, 2002). Moreover, cubilin too binds amnionless, a poly peptide that is necessary for the expression of cubilin on the jail cell membrane (Amsellem et al., 2010). Cubilin, in conjunction with megalin, has an essential office in the uptake of albumin (i.e., reabsorption) by the proximal tubules of the kidneys (Zhai et al., 2000; Amsellem et al., 2010). Cubilin- and/or megalin-deficiency in mice and dogs was shown to cause a decrease in the uptake of albumin in the proximal tubule resulting in albuminuria (Birn et al., 2000; Amsellem et al., 2010). Additionally, patients with Imerslund-Gräsbeck syndrome, acquired by a mutation in the cubilin gene, in general suffer from proteinurea, demonstrating the importance of cubilin in protein renal reabsorption (Grasbeck, 2006).
Albumin as a Drug Carrier in Oncology
Considering the enhanced permeation and retentiveness consequence and the accumulation of albumin in the tumor interstitium, the development of albumin equally a drug carrier is increasingly of import to consider in terms of the targeted delivery of cancer therapy (Kratz, 2008, 2010). It has as well been proposed that albumin carriers take reward of the presence of albondin on the endothelium and SPARC in the tumor interstitium to increase the accumulation of drugs in the tumor infinite (Desai et al., 2009; Kratz, 2010). Various drug delivery systems with albumin have been adult including albumin nanoparticles, drug albumin conjugates, albumin-binding drug derivatives and prodrugs (for reviews see Kratz, 2008, 2010).
The development and market approving of the paclitaxel-loaded albumin nanoparticle, nab-paclitaxel or Abraxane®, was a major breakthrough in the field of albumin carrier development. Abraxane® was initially approved for clinical employ in the United States in 2005 (Kudlowitz and Muggia, 2014). This albumin nanoparticle is indicated for the treatment of metastatic breast cancer, after failure of combination chemotherapy (Kudlowitz and Muggia, 2014). More recently, Abraxane® has as well been described for the first-line treatment of patients with metastatic adenocarcinoma of the pancreas, in combination with gemcitabine, and patients with locally avant-garde or metastatic non-minor cell lung carcinoma, in combination with carboplatin (Kudlowitz and Muggia, 2014). Abraxane® has a greater therapeutic alphabetize than paclitaxel alone, beingness administered at higher doses with less toxicity and more efficacy than traditional paclitaxel therapy (Gradishar et al., 2005; Socinski et al., 2012; Iwamoto, 2013). Abraxane® is currently nevertheless being further evaluated in clinical trials for other tumors, such as cancer of the float (NCT00583349) and multiple myeloma (NCT02075021).
Moreover, albumin-binding as a general strategy for improving the pharmacokinetics of drugs is too being assessed. Traditionally, the binding of a drug to albumin is believed to reduce the level of costless drug available to exert its therapeutic activity (Lancon et al., 2004; Vuignier et al., 2010). However, studies have besides demonstrated mechanisms by which albumin acts to effectively improve therapeutic use or reduce rapid clearance (Dennis et al., 2002; Merlot and Richardson, 2014). For case, the experimental anti-cancer thiosemicarbazone, namely di-2-pyridylketone iv,4-dimethyl-3-thiosemicarbazone (Dp44mT) (Merlot et al., 2013a), has been shown to be internalized by cancer cells via a putative carrier/receptor (Merlot et al., 2013b; Merlot and Richardson, 2014). Interestingly, the uptake, toxicity and apoptotic activity of Dp44mT is greatly enhanced in the presence of HSA (Merlot and Richardson, 2014). Considering Dp44mT targets lysosomes to induce apoptosis (Lovejoy et al., 2011), and that HSA potentially undergoes lysosomal catabolism in tumors (Andersson et al., 1991; Stehle et al., 1997), it can be hypothesized that HSA facilitates Dp44mT commitment to the lysosomes, enhancing its anti-cancer activity (Merlot and Richardson, 2014). Although studies are yet to identify the exact machinery of the HSA-stimulated uptake procedure, albumin-binding may provide an advantage when generating tumor targeting agents and this requires farther intense investigation.
Decision
Albumin is a versatile and captivating protein. In view of the large confinement of albumin within the vascular and interstitial space, the intracellular distribution of albumin has remained poorly characterized for many years. It may be possible that albumin, under specific atmospheric condition or during cellular stress, is taken upwardly by normal cells and tumor cells at depression and high levels, respectively, due to their metabolic rate. The exact role of some incompletely characterized albumin-bounden proteins (i.e., hnRNPs and calreticulin) in mediating albumin uptake remains to be determined. Still, the search and characterization of albumin-binding proteins, particularly in cancer cells, is of considerable involvement in light of the development of albumin every bit an effective drug carrier to target tumors.
Disharmonize of Interest Argument
The authors declare that the research was conducted in the absenteeism of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Angelica G. Merlot is the recipient of an Early Career Research Grant from the University of Sydney. Des R. Richardson is the recipient of a National Wellness and Medical Research Council (NHMRC) Senior Principal Research Fellowship and Project Grants. Danuta S. Kalinowski is the recipient of a NHMRC Projection Grant (1048972) and a Helen and Robert Ellis Fellowship from the Sydney Medical School Foundation of The University of Sydney.
References
Amsellem, S., Gburek, J., Hamard, Thou., Nielsen, R., Willnow, T. E., Devuyst, O., et al. (2010). Cubilin is essential for albumin reabsorption in the renal proximal tubule. J. Am. Soc. Nephrol. 21, 1859–1867. doi: 10.1681/ASN.2010050492
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Andersen, J. T., Dalhus, B., Cameron, J., Daba, Yard. B., Plumridge, A., Evans, 50., et al. (2012). Structure-based mutagenesis reveals the albumin-binding site of the neonatal Fc receptor. Nat. Commun. 3, 610. doi: ten.1038/ncomms1607
Pubmed Abstract | Pubmed Total Text | CrossRef Full Text
Anderson, C. L., Chaudhury, C., Kim, J., Bronson, C. L., Wani, M. A., and Mohanty, S. (2006). Perspective—FcRn transports albumin: relevance to immunology and medicine. Trends Immunol. 27, 343–348. doi: 10.1016/j.it.2006.05.004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Andersson, C., Iresjo, B. One thousand., and Lundholm, K. (1991). Identification of tissue sites for increased albumin degradation in sarcoma-begetting mice. J. Surg. Res. 50, 156–162. doi: x.1016/0022-4804(91)90240-M
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Birn, H., Fyfe, J. C., Jacobsen, C., Mounier, F., Verroust, P. J., Orskov, H., et al. (2000). Cubilin is an albumin binding protein of import for renal tubular albumin reabsorption. J. Clin. Invest. 105, 1353–1361. doi: 10.1172/JCI8862
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Chaudhury, A., Chander, P., and Howe, P. H. (2010). Heterogeneous nuclear ribonucleoproteins (hnRNPs) in cellular processes: focus on hnRNP E1'south multifunctional regulatory roles. RNA 16, 1449–1462. doi: ten.1261/rna.2254110
Pubmed Abstract | Pubmed Total Text | CrossRef Total Text
Chaudhury, C., Mehnaz, Southward., Robinson, J. M., Hayton, W. Fifty., Pearl, D. K., Roopenian, D. C., et al. (2003). The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J. Exp. Med. 197, 315–322. doi: ten.1084/jem.20021829
Pubmed Abstract | Pubmed Full Text | CrossRef Total Text
Commisso, C., Davidson, S. M., Soydaner-Azeloglu, R. G., Parker, S. J., Kamphorst, J. J., Hackett, Southward., et al. (2013). Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 497, 633–637. doi: 10.1038/nature12138
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Cui, Due south., Verroust, P. J., Moestrup, S. K., and Christensen, E. I. (1996). Megalin/gp330 mediates uptake of albumin in renal proximal tubule. Am. J. Physiol. 271, F900–F907. doi: ten.1038/nature12138
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Dennis, M. South., Zhang, Thousand., Meng, Y. G., Kadkhodayan, M., Kirchhofer, D., Combs, D., et al. (2002). Albumin bounden equally a full general strategy for improving the pharmacokinetics of proteins. J. Biol. Chem. 277, 35035–35043. doi: 10.1074/jbc.M205854200
Pubmed Abstract | Pubmed Total Text | CrossRef Full Text
Desai, N., Trieu, 5., Damascelli, B., and Soon-Shiong, P. (2009). SPARC expression correlates with tumor response to albumin-jump paclitaxel in head and neck cancer patients. Transl. Oncol. 2, 59–64. doi: x.1593/tlo.09109
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Desai, N. P., Trieu, V., Hwang, L. Y., Wu, R., Before long-Shiong, P., and Gradishar, W. J. (2008). Improved effectiveness of nanoparticle albumin-spring (nab) paclitaxel versus polysorbate-based docetaxel in multiple xenografts as a role of HER2 and SPARC status. Anticancer Drugs 19, 899–909. doi: 10.1097/CAD.0b013e32830f9046
Pubmed Abstruse | Pubmed Full Text | CrossRef Full Text
Evans, T. W. (2002). Review article: albumin equally a drug–biological effects of albumin unrelated to oncotic pressure. Aliment. Pharmacol. Ther. 16(Suppl. 5), 6–11. doi: 10.1046/j.1365-2036.2002.00190.10
Pubmed Abstruse | Pubmed Full Text | CrossRef Full Text
Fritzsche, T., Schnolzer, M., Fiedler, Due south., Weigand, Grand., Wiessler, M., and Frei, E. (2004). Isolation and identification of heterogeneous nuclear ribonucleoproteins (hnRNP) from purified plasma membranes of human being tumour cell lines as albumin-binding proteins. Biochem. Pharmacol. 67, 655–665. doi: 10.1016/j.bcp.2003.09.027
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Ghinea, Due north., Eskenasy, M., Simionescu, Grand., and Simionescu, North. (1989). Endothelial albumin binding proteins are membrane-associated components exposed on the prison cell surface. J. Biol. Chem. 264, 4755–4758.
Pubmed Abstract | Pubmed Full Text
Ghinea, Northward., Fixman, A., Alexandru, D., Popov, D., Hasu, M., Ghitescu, L., et al. (1988). Identification of albumin-binding proteins in capillary endothelial cells. J. Cell Biol. 107, 231–239.
Pubmed Abstruse | Pubmed Full Text
Gradishar, Westward. J., Tjulandin, S., Davidson, North., Shaw, H., Desai, N., Bhar, P., et al. (2005). Stage III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J. Clin. Oncol. 23, 7794–7803. doi: 10.1200/JCO.2005.04.937
Pubmed Abstruse | Pubmed Full Text | CrossRef Full Text
Greish, K. (2007). Enhanced permeability and retentiveness of macromolecular drugs in solid tumors: a royal gate for targeted anticancer nanomedicines. J. Drug Target. 15, 457–464. doi: 10.1080/10611860701539584
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Iancu, C., Mocan, L., Bele, C., Orza, A. I., Tabaran, F. A., Catoi, C., et al. (2011). Enhanced laser thermal ablation for the in vitro treatment of liver cancer by specific delivery of multiwalled carbon nanotubes functionalized with homo serum albumin. Int. J. Nanomed. 6, 129–141. doi: 10.2147/IJN.S15841
Pubmed Abstruse | Pubmed Full Text | CrossRef Total Text
Iwamoto, T. (2013). Clinical awarding of drug delivery systems in cancer chemotherapy: review of the efficacy and side furnishings of approved drugs. Biol. Pharm. Bull. 36, 715–718. doi: 10.1248/bpb.b12-01102
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Kudlowitz, D., and Muggia, F. (2014). Nanoparticle albumin-spring paclitaxel (nab-paclitaxel): extending its indications. Expert Opin. Drug Saf. 13, 681–685. doi: ten.1517/14740338.2014.910193
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Lancon, A., Delmas, D., Osman, H., Thenot, J. P., Jannin, B., and Latruffe, N. (2004). Human hepatic cell uptake of resveratrol: involvement of both passive improvidence and carrier-mediated procedure. Biochem. Biophys. Res. Comm. 316, 1132–1137. doi: ten.1016/j.bbrc.2004.02.164
Pubmed Abstract | Pubmed Full Text | CrossRef Total Text
Lovejoy, D. B., Jansson, P. J., Brunk, U. T., Wong, J., Ponka, P., and Richardson, D. R. (2011). Antitumor activeness of metal-chelating compound Dp44mT is mediated by formation of a redox-active copper complex that accumulates in lysosomes. Cancer Res. 71, 5871–5880. doi: ten.1158/0008-5472.Tin can-11-1218
Pubmed Abstract | Pubmed Total Text | CrossRef Full Text
Maeda, H., Wu, J., Sawa, T., Matsumura, Y., and Hori, K. (2000). Tumor vascular permeability and the EPR upshot in macromolecular therapeutics: a review. J. Command. Release 65, 271–284. doi: 10.1016/S0168-3659(99)00248-5
Pubmed Abstract | Pubmed Total Text | CrossRef Full Text
Matsumura, Y., and Maeda, H. (1986). A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 46, 6387–6392.
Pubmed Abstruse | Pubmed Full Text
Mendlovic, F., and Conconi, Grand. (2010). Calreticulin: a multifaceted protein. Nature Educ. 4, 1.
Merlot, A. One thousand., Pantarat, North., Menezes, S. Five., Sahni, S., Richardson, D. R., and Kalinowski, D. S. (2013b). Cellular uptake of the antitumor agent Dp44mT occurs via a carrier/receptor-mediated mechanism. Mol. Pharmacol. 84, 911–924. doi: 10.1124/mol.113.088393
Pubmed Abstract | Pubmed Full Text | CrossRef Total Text
Merlot, A. M., and Richardson, D. R. (2014). Receptor recognition and lysosomal targeting to enhance cytotoxicity of novel anti-cancer agents that bind iron and copper. Vitam. Miner. 3:e125. doi: 10.4172/vms.1000e125
CrossRef Total Text
Milici, A. J., Watrous, North. Eastward., Stukenbrok, H., and Palade, Yard. East. (1987). Transcytosis of albumin in capillary endothelium. J. Cell Biol. 105, 2603–2612.
Pubmed Abstract | Pubmed Full Text
Moestrup, Due south. K., Kozyraki, R., Kristiansen, M., Kaysen, J. H., Rasmussen, H. H., Brault, D., et al. (1998). The intrinsic factor-vitamin B12 receptor and target of teratogenic antibodies is a megalin-binding peripheral membrane poly peptide with homology to developmental proteins. J. Biol. Chem. 273, 5235–5242. doi: 10.1074/jbc.273.ix.5235
Pubmed Abstruse | Pubmed Full Text | CrossRef Full Text
Neesse, A., Frese, Chiliad. Chiliad., Chan, D. Due south., Bapiro, T. E., Howat, W. J., Richards, F. M., et al. (2014). SPARC independent drug delivery and antitumour furnishings of nab-paclitaxel in genetically engineered mice. Gut 63, 974–983. doi: 10.1136/gutjnl-2013-305559
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Ober, R. J., Martinez, C., Lai, 10., Zhou, J., and Ward, E. South. (2004). Exocytosis of IgG every bit mediated by the receptor, FcRn: an assay at the single-molecule level. Proc. Natl. Acad. Sci. The statesA. 101, 11076–11081. doi: 10.1073/pnas.0402970101
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Peters, T. (1996). All Almost Albumin: Biochemistry, Genetics and Medical Applications. San Diego, CA: Academic Press Limited.
Peterson, H. I., and Appelgren, 1000. L. (1973). Experimental studies on the uptake and rentention of labelled proteins in a rat tumour. Eur. J. Cancer 9, 543–547. doi: 10.1016/0014-2964(73)90142-4
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Podhajcer, O. L., Benedetti, L. 1000., Girotti, M. R., Prada, F., Salvatierra, E., and Llera, A. S. (2008). The role of the matricellular protein SPARC in the dynamic interaction betwixt the tumor and the host. Cancer Metastasis Rev. 27, 691–705. doi: 10.1007/s10555-008-9146-7
CrossRef Full Text
Porter, P. L., Sage, E. H., Lane, T. F., Funk, S. E., and Gown, A. M. (1995). Distribution of SPARC in normal and neoplastic human tissue. J. Histochem. Cytochem. 43, 791–800. doi: ten.1177/43.viii.7622842
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Sarav, M., Wang, Y., Hack, B. Thousand., Chang, A., Jensen, 1000., Bao, 50., et al. (2009). Renal FcRn reclaims albumin but facilitates elimination of IgG. J. Am. Soc. Nephrol. twenty, 1941–1952. doi: ten.1681/ASN.2008090976
Pubmed Abstract | Pubmed Full Text | CrossRef Total Text
Schilling, U., Friedrich, E. A., Sinn, H., Schrenk, H. H., Clorius, J. H., and Maier-Borst, W. (1992). Pattern of compounds having enhanced tumour uptake, using serum albumin every bit a carrier–Part II. In vivo studies. Int. J. Rad. Appl. Instrum. B 19, 685–695. doi: ten.1016/0883-2897(92)90103-6
Pubmed Abstruse | Pubmed Total Text | CrossRef Full Text
Schnitzer, J. E. (1992). gp60 is an albumin-binding glycoprotein expressed by continuous endothelium involved in albumin transcytosis. Am. J. Physiol. 262, H246–H254. doi: ten.1016/1050-1738(93)90012-U
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Schnitzer, J. East., Allard, J., and Oh, P. (1995). NEM inhibits transcytosis, endocytosis, and capillary permeability: implication of caveolae fusion in endothelia. Am. J. Physiol. 268, H48–H55.
Pubmed Abstract | Pubmed Full Text
Schnitzer, J. Due east., and Bravo, J. (1993). Loftier analogousness binding, endocytosis, and degradation of conformationally modified albumins. Potential role of gp30 and gp18 as novel scavenger receptors. J. Biol. Chem. 268, 7562–7570.
Pubmed Abstract | Pubmed Full Text
Schnitzer, J. E., Carley, W. W., and Palade, Thou. E. (1988). Albumin interacts specifically with a 60-kDa microvascular endothelial glycoprotein. Proc. Natl. Acad. Sci. U.s.A. 85, 6773–6777. doi: 10.1073/pnas.85.xviii.6773
Pubmed Abstruse | Pubmed Full Text | CrossRef Full Text
Schnitzer, J. East., and Oh, P. (1992). Antibodies to SPARC inhibit albumin binding to SPARC, gp60, and microvascular endothelium. Am. J. Physiol. 263, H1872–H1879.
Pubmed Abstruse | Pubmed Total Text
Schnitzer, J. Eastward., and Oh, P. (1994). Albondin-mediated capillary permeability to albumin. Differential office of receptors in endothelial transcytosis and endocytosis of native and modified albumins. J. Biol. Chem. 269, 6072–6082.
Pubmed Abstract | Pubmed Full Text
Schnitzer, J. E., Sung, A., Horvat, R., and Bravo, J. (1992). Preferential interaction of albumin-binding proteins, gp30 and gp18, with conformationally modified albumins. Presence in many cells and tissues with a possible role in catabolism. J. Biol. Chem. 267, 24544–24553.
Pubmed Abstruse | Pubmed Full Text
Seetharam, B., Christensen, East. I., Moestrup, S. K., Hammond, T. Chiliad., and Verroust, P. J. (1997). Identification of rat yolk sac target protein of teratogenic antibodies, gp280, every bit intrinsic cistron-cobalamin receptor. J. Clin. Invest. 99, 2317–2322. doi: 10.1172/JCI119411
Pubmed Abstract | Pubmed Full Text | CrossRef Total Text
Sinn, H., Schrenk, H. H., Friedrich, E. A., Schilling, U., and Maier-Borst, Westward. (1990). Design of compounds having an enhanced neoplasm uptake, using serum albumin as a carrier. Role I. Int. J. Rad. Appl. Instrum. B 17, 819–827. doi: 10.1016/0883-2897(92)90103-6
Pubmed Abstract | Pubmed Total Text | CrossRef Total Text
Socinski, M. A., Bondarenko, I., Karaseva, N. A., Makhson, A. M., Vynnychenko, I., Okamoto, I., et al. (2012). Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J. Clin. Oncol. xxx, 2055–2062. doi: 10.1200/JCO.2011.39.5848
Pubmed Abstract | Pubmed Total Text | CrossRef Full Text
Stehle, Grand., Sinn, H., Wunder, A., Schrenk, H. H., Stewart, J. C., Hartung, G., et al. (1997). Plasma protein (albumin) catabolism by the tumor itself–implications for tumor metabolism and the genesis of cachexia. Crit. Rev. Oncol. Hematol. 26, 77–100. doi: x.1016/S1040-8428(97)00015-2
Pubmed Abstruse | Pubmed Total Text | CrossRef Total Text
Sudlow, G., Birkett, D. J., and Wade, D. N. (1976). Further label of specific drug binding sites on human serum albumin. Mol. Pharmacol. 12, 1052–1061.
Pubmed Abstract | Pubmed Full Text
Sugio, S., Kashima, A., Mochizuki, S., Noda, One thousand., and Kobayashi, K. (1999). Crystal structure of homo serum albumin at two.v A resolution. Protein Eng. 12, 439–446. doi: 10.1093/protein/12.six.439
Pubmed Abstract | Pubmed Total Text | CrossRef Full Text
Tiruppathi, C., Finnegan, A., and Malik, A. B. (1996). Isolation and characterization of a jail cell surface albumin-binding protein from vascular endothelial cells. Proc. Natl. Acad. Sci. UsA. 93, 250–254. doi: 10.1073/pnas.93.i.250
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Tiruppathi, C., Song, West., Bergenfeldt, K., Sass, P., and Malik, A. B. (1997). Gp60 activation mediates albumin transcytosis in endothelial cells by tyrosine kinase-dependent pathway. J. Biol. Chem. 272, 25968–25975. doi: 10.1074/jbc.272.41.25968
Pubmed Abstract | Pubmed Total Text | CrossRef Full Text
van der Veldt, A. A., Luurtsema, One thousand., Lubberink, M., Lammertsma, A. A., and Hendrikse, N. H. (2008). Individualized treatment planning in oncology: role of PET and radiolabelled anticancer drugs in predicting neoplasm resistance. Curr. Pharm. Des. 14, 2914–2931. doi: ten.2174/138161208786404344
Pubmed Abstract | Pubmed Full Text | CrossRef Total Text
Vuignier, Chiliad., Schappler, J., Veuthey, J. L., Carrupt, P. A., and Martel, S. (2010). Drug-poly peptide binding: a disquisitional review of analytical tools. Anal. Bioanal. Chem. 398, 53–66. doi: 10.1007/s00216-010-3737-i
Pubmed Abstruse | Pubmed Full Text | CrossRef Full Text
Wang, J., Ueno, H., Masuko, T., and Hashimoto, Y. (1994). Bounden of serum albumin on tumor cells and characterization of the albumin binding protein. J. Biochem. 115, 898–903.
Pubmed Abstract | Pubmed Full Text
Wunder, A., Stehle, G., Sinn, H., Schrenk, H., Hoffbiederbeck, D., Bader, F., et al. (1997). Enhanced albumin uptake by rat tumors. Int. J. Oncol. 11, 497–507. doi: 10.3892/ijo.11.3.497
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Yamagishi, T., Sahni, Southward., Precipitous, D. K., Arvind, A., Jansson, P. J., and Richardson, D. R. (2013). P-glycoprotein mediates drug resistance via a novel machinery involving lysosomal sequestration. J. Biol. Chem. 288, 31761–31771. doi: 10.1074/jbc.M113.514091
Pubmed Abstruse | Pubmed Full Text | CrossRef Full Text
Zhai, X. Y., Nielsen, R., Birn, H., Drumm, K., Mildenberger, S., Freudinger, R., et al. (2000). Cubilin- and megalin-mediated uptake of albumin in cultured proximal tubule cells of opossum kidney. Kidney Int. 58, 1523–1533. doi: 10.1046/j.1523-1755.2000.00314.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Source: https://www.frontiersin.org/articles/10.3389/fphys.2014.00299/full
0 Response to "Review Modifications of Human Serum Albumin and Their Binding Effect"
Post a Comment